
Verimed Africa
Verimed Africa Pty (Ltd) was established in July 2010 to act as an independent pathology/radiology risk manager for medical funders and administrators. Verimed Africa has developed an IT solution which verifies the correctness of pathology/radiology accounts.

Verimed Africa
We have identified a number of areas where erroneous billing and over-servicing are performed by Radiology and Pathology practices.
We Are Health Fund Risk Managers
- MRI (magnetic resonance imaging)
- CT (computed tomography)
- PET (positron emission tomography)
- Interventional radiology
- Ultrasound
- Nuclear medicine
- Digital mammography
- Molecular imaging
- General black and white Xrays
- Andrology
- Bacteriology
- Biochemical tests
- Cytology
- Haematology
- Histology
- Human Genetics
- Immunology
- Microbiology
- Serology
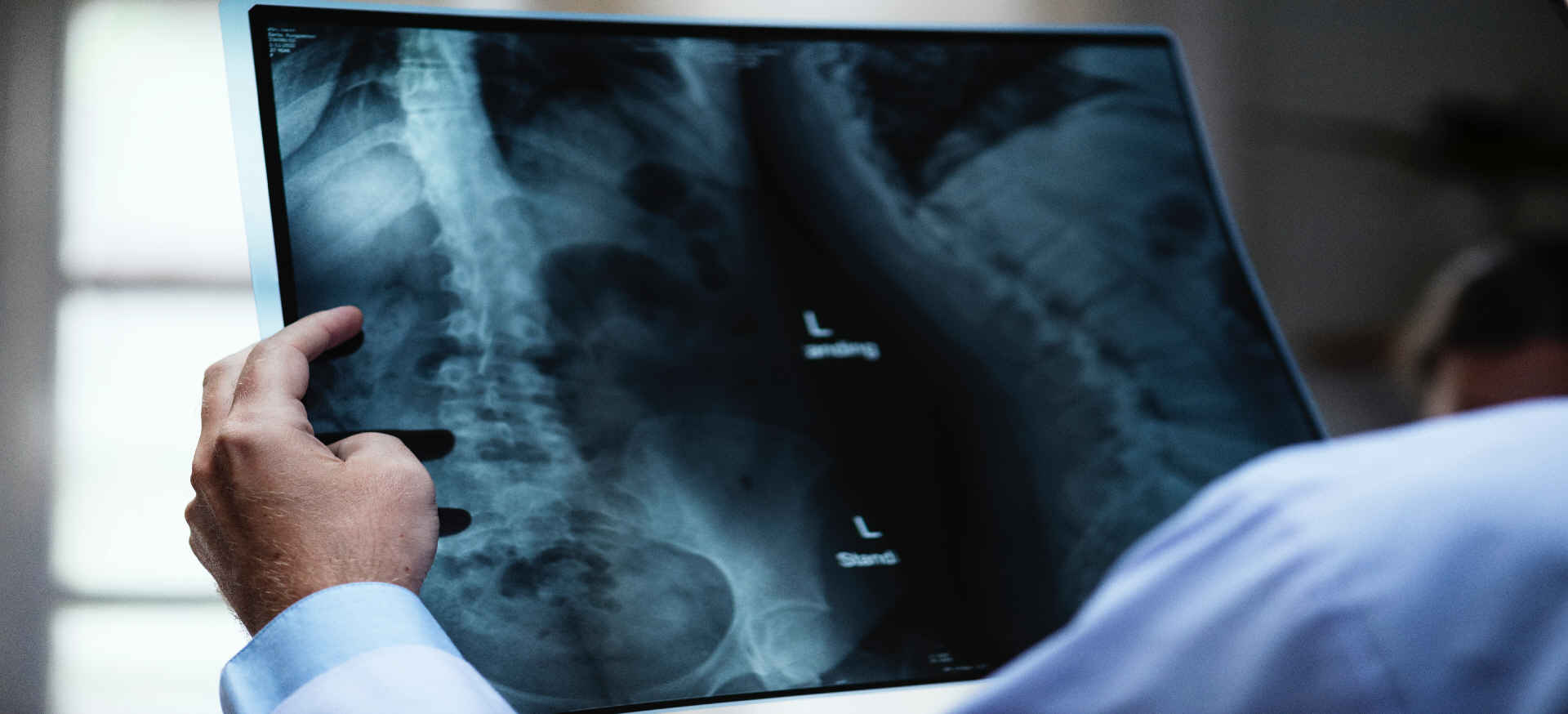
Radiology and Pathology utilization management has become a top priority for Medical Schemes as verification of these accounts eliminates duplicate submissions and ensures correct billing practice. The main objective of Verimed Africa is to offer Radiology and Pathology Management Service to Medical Schemes in order to reduce their expenditure in this field that comprises roughly 11% of Medical Scheme spend.
About us
The owners and directors of Verimed Africa (Pty) Ltd are Chris Adams, Paul Horn and Arthur Moore. The directors are all South African citizens.
Chris Adams is a highly qualified and experienced pathology laboratory manager with 18 years experience in a major pathology practice in Johannesburg South Africa. Prior to joining the private sector he was employed at the South African Institute for Medical Research. Adams is a member of the SAMLTS and is a Medical Technologist - Clinical Pathology, Chemical Pathology, he holds a B Comm degree (UNISA), Post Graduate Diploma Health Economics (UCT) and an MBA from Brunel University (U.K.) / Henley Management College.
Paul Horn has a BSc in Mathematics and Statistics and has 15 years experience in Commercial IT development.
Arthur Moore has been a Medical Technologist for over 30 years, 19 of which were in a Pathology Laboratory managerial position, before becoming a co-founder of Verimed Africa (Pty) LTD. He Has resided in Botswana for 17 years and has two Diplomas in Medical Technology – Chemical Pathology and Clinical Pathology.
Verimed Africa employs a Consultant Radiologist and Pathologist together with qualified radiographers and Medical Technologists for the assessing and verification of radiology and pathology accounts. All have extensive experience in both private practices and in an administrative or consulting capacity with various Medical Schemes/Administrators or Managed Healthcare Companies. An IT Systems Product has been developed, the rules of which are based on a combination of: -
- RSSA
- NHRPL
- ICD10 coding
- DoH
- CMS
- HPCSA
- NPG
- BHF
- SAMA
Verimed Africa has identified a number of areas where erroneous billing and over-servicing are performed by Radiology and Pathology practices:
 Duplicate submissions and/or payments
Duplicate submissions and/or payments
 Up-coding of tariffs
Up-coding of tariffs
 Tariff combinations
Tariff combinations
 Comparison views
Comparison views
 Unbundled Group Tests
Unbundled Group Tests
 Overcharging of consumables and contrast medium
Overcharging of consumables and contrast medium
 Inappropriate tariffs
Inappropriate tariffs
 Increased utilization
Increased utilization
 Elective medicine
Elective medicine
Useful information
Radiology examinations
Excessive radiology examinations not only lead to being exposed to excessive amounts of radiation, but also increase your medical accounts.
Radiology examinations may not be funded by your Medical Scheme if performed without a doctors referral letter.
Specialists, ie. Neurosurgeons, Orthopaedic surgeons etc. Requests specific examinations, thus basic x-rays may be deemed redundant as they usually have this information, if it’s not your first visit to the Specialist. Also, if you have had the standard x-ray previously, and your GP refers you to the Specialist – there is no need for the standard x-rays to be repeated. Advise your Specialist that standard x-rays have been done, and he/she may be aware to only request specialized views.
Radiology Practices have radiology protocols of standard views which they perform for particular areas of interest.
For Example:
Standard views for first time visit referred by a General Practitioner for Lower Back x-rays (Lumbar Spine x-rays) are 3 views of the spine. Some practices add a pelvis as well as flexion and extension without those views being requested. A more common example is a GP adding extra knee views that should only be requested by an orthopaedic surgeon.
The Radiology Request Form is the legal document submitted to the Scheme for assessing claims. The information on these request forms drives the payment of such claims. Should any add on examinations be requested by yourself or additional examinations performed by the Radiology Practice that are not motivated for or deemed necessary, payment may be declined by the Scheme.
Excessive radiation is harmful. Ask your doctor to be specific with the x-ray requests in order to prevent unnecessary irradiation and excessive medical claims.
Pathology Testing
Remember that excess and unnecessary testing increases the account. This account is either your responsibility or that of your medical aid. The costs if borne by the medical aid are eventually paid for by you via increased contributions.
When your Doctor orders lab tests - Ask them how much this should cost - remember its your money! Ask if all of these tests are necessary?
Ask if your Doctor if he/she receives any financial advantage as a result of this referral?
Ask why the Doctor prefers a certain Laboratory?
There are Medical Technologist laboratories available that charge 26% less for tests. In addition these labs are not allowed to perform "shopping lists of tests", these profiles increase the costs of pathology testing.
Have you had these tests recently, if so must they be repeated? When you are referred from a GP to a specialist ask the GP to send all relevant lab results with the referral. This may prevent unnecessary and costly repeats. Point out to the specialist that you have previously had lab tests performed.
Ask the lab to give you a copy of the request form when they take the blood or other specimen for your tests. This request form should be designed according to National Pathology Group guidelines. Some Pathology labs may automatically add on extra tests to those that the Doctor ordered. Some Pathology labs may not adhere to the profiles as set by the National Pathology Group.
If the account arrives and the amount is different from that which your Doctor indicated then ask your Doctor to explain the difference. If necessary the Doctor should phone the laboratory to confirm the account.
Tumour Markers and Screening for Cancer
Screening and early diagnosis of cancer have intuitive appeal to anyone that has dealt with patients with incurable cancer. Screening tests include fecal occult blood, mammography, cervical Pap smears and blood tumour markers. Blood tests for numerous tumour markers are commercially available and their use has recently been comprehensively reviewed.
The ease of obtaining a sample and the spectrum of organ related tumour markers available to the clinician, has led to a surge in tumour marker usage that may be inappropriate.
The only blood tumour marker that is accepted by most authorities to have any role in screening for malignancy is the PSA test. This is however not accepted without controversy and Law even states, "…the one certainty about prostate specific antigen testing is that it causes harm."
The role of tumour markers such as CA 15-3, CA19-9, CA125, CEA etc. for cancer screening are even more tenuous. By way of a specific example, CA15-3 is touted by some as a "test for breast cancer" and has the following performance characteristics : "… 5.5% of 1050 normal subjects…..23% of patients with primary breast cancer……69% with metastatic breast cancer……other malignancies, including pancreatic (80%)….benign breast diseases (16%)…should not be used to diagnose primary breast cancer…most useful in monitoring therapy and disease progression…."
It is generally accepted that , with the exception of PSA, none of the other blood tumour markers has any role in the screening, or diagnosis of malignancy. An additional factor to consider is the potential harm inflicted on patients by false reassurance (77% of primary breast cancer will be missed by CA15-3) and the emotional distress as well as unnecessary further testing on 5.5% of patients without any malignancy (false positives). This scenario holds true for any of the other blood tumour markers mentioned above.
In conclusion there is no evidence to support the routine use of blood tumour markers as a screening or diagnostic aid. The inappropriate use of blood tumour markers must be actively discouraged.
Dr CJ Pretorius MB ChB, FC Path (Chem) SA
1.Sturgeon C, Practice Guidelines for Tumour Marker Use in the Clinic (Review), Clinical Chemistry, 48:1151 - 59, 2002 2.Law M, Screening without evidence of efficacy (Editorial), BMJ, 328:301 - 2, 2004 3.Burtis CA Ashwood ER, Tietz Textbook of Clinical Chemistry, 2nd edition; 916 - 7
Laboratory monitoring of Diabetes Millitus
Monitoring of Diabetes Mellitus - the role of HbA1c
Terminology:
DM Diabetes Mellitus GH Glycated haemoglobin HbA1c Haemoglobin A1c DCCT Diabetes Control and Complications Trial NGSP National Glycohemoglobin Standardization Program HPLC High Performance Liquid Chromatography
Introduction:
Diabetes Mellitus (DM) is a chronic, systemic disease characterised by disturbed carbohydrate metabolism, micro vascular complications (retina and kidney) and macro vascular complications (coronary and carotid arteries). To put the costs associated with DM into perspective, it has been reported that the annual per capita healthcare cost for a diabetic is 4 times higher than for a non-diabetic person. The costs attributable to DM arise because of both acute and chronic complications. It has been conclusively established that micro vascular complications can be decreased in diabetics who are intensively managed, compared to a control group who are less well controlled.
Glycated haemoglobin is established as the laboratory parameter that reflects the degree of control of a diabetic person. Although glycated haemoglobin (GH) and Haemoglobin A1c (HbA1c) are often used interchangeably, it should be pointed out that HbA1c is a component of GH. The HbA1c level in a person reflects an integrated assessment of the mean glycaemic control over the average red cell life span for an individual.
HbA1c was the main laboratory parameter used to reflect glycaemic control in the DCCT1 study. Subsequently HbA1c levels exceeding 8.1% was shown to be associated with a dramatic rise in microalbuminuria and it was recommended that by controlling HbA1c levels below 8.1%, the incidence of diabetic nephropathy could be reduced. Recently exiting evidence that macro vascular complications are reduced in well-controlled diabetics compared to poorly controlled diabetics was published . The thickness of the carotid artery intima-media, a reflection of atherosclerosis, was related to HbA1c level. Good control of DM (measured by normal HbA1c levels) led to a reduced progression of disease.
Laboratory aspects of measuring glycated haemoglobin:
The irreversible binding of glucose molecules to haemoglobin molecules causes an alteration of the charge, chemical binding property and immunological characteristics of the haemoglobin molecule. Ion exchange chromatography, affinity chromatography and immuno-assay methodology can be used to separate the glycated haemoglobin from the non-glycated haemoglobin. The results of the GH are expressed as a percentage of total haemoglobin. HbA1c refers to a particular fraction of GH and most assays reports are standardised to HbA1c irrespective of whether the total GH or HbA1c fraction is measured directly.
The degree of glycation of haemoglobin is a function of the average glucose concentration and the life span of the red cells. In haemolytic conditions the red cell life span is shortened and the GH is lower for any integrated glucose concentration than in a comparable individual with normal (120 days) red cell survival.
In a comprehensive review on laboratory monitoring of diabetic patients, under the auspices of the National Association of Clinical Biochemistry and the American Diabetes Association, it is strongly recommended that assays for GH (HbA1c) must be approved by a special task group (NGSP) and that the results must be related to DCCT equivalent results. Diagnostic manufacturers submit their assays on a yearly basis for approval and the results are published (http://www.missouri.edu/~diabetes/ngsp.html) - a copy of a list of NGSP certified methods are attached as a supplement to this document)
The bulk of methodologies approved by the NGSP are chromatographical and immunological methods. The chromatographical methods are mostly performed on dedicated, automated high performance liquid chromatography (HPLC) analysers. The immunological assays are performed by turbidimetric methodology on routine laboratory analysers. Comparative evaluations of methodology studies show no significant difference in the performance of automated HPLC and immunological assays.
Clinical aspects of measuring glycated haemoglobin5:
HbA1c measurements are the recommended assay for monitoring glycaemic control. Other glycated proteins such as Fructosamine (glycated albumin) are not recommended for routine monitoring.
HbA1c assays are recommended twice a year for well-controlled diabetics and quarterly (4 times a year) in poorly controlled diabetics.
HbA1c is not recommended for screening or diagnosis of diabetes mellitus. Plasma glucose is the sole diagnostic criterion for diabetes mellitus.
In persons with decreased red cell survival (acute blood loss or abnormal haemoglobin) the HbA1c values will be falsely lowered regardless of the method used to measure HbA1c (HPLC or immunological). In populations with a high incidence of abnormal haemoglobins an alternative glycated protein assay such as Fructosamine should be considered .
Discussion:
The assertion that HPLC represents the "gold standard" for measuring HbA1c and therefore that HPLC should replace immunological assays for HbA1c is not apparent from a review of the literature. What is clear is that both HPLC and immunological methodologies should be standardised to a traceable international reference standard, such as NGSP certified methodology.
The immunological assays that feature in the NGSP certification list are in common use in South African laboratories. These assays are generally billed under code 4182 (Turbidimetric or nepeholometric methodology) at a 2004 NRPL tariff of R 55.10.
While the HPLC methodology is undoubtedly acceptable as an alternative to immunological assays on technical grounds, it is questionable whether the HPLC methodology should be reimbursed at a higher rate, as an acceptable alternative methodology at a lower price is available. If the principle of technological up coding is accepted by medical aid funders, it can be confidently predicted that this will be the start of an avalanche.
Recommendations:
1. HbA1c methods should be NGSP certified.
2. Reimbursement should be at the level of the lowest cost NGSP certified HbA1c method - in this case code 4182.
3. The principle of technological up coding should be resisted.
Technological up coding - where a more expensive methodology to measure an analyte is used although no clinical benefit is apparent.
1. American Diabetes Association. Economic consequences of diabetes mellitus in the US in 1997. Diabetes Care. 1998;21:296 - 308
2. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependant diabetes, NEJM. 1993; 329;977 - 986
3. Krolewski SA et al. Glycoslated hem
